Strategie avanzate di Process Validation: l’impegno di Microchem per la conformità e la qualità
Process Validation in Microchem: precisione e personalizzazione nel rispetto delle normative

Process Validation in Microchem: precisione e personalizzazione nel rispetto delle normative
Ampliamento e innovazione nel cuore produttivo di Microchem
Affrontare con successo le complessità regolatorie e di qualità: l'expertise unica di Microchem nel mondo della micronizzazione.
Come Microchem supporta i clienti nel processo di autorizzazione dei prodotti micronizzati.
Esplora come Microchem eccelle in Quality Assurance e Regulatory Support, offrendo soluzioni su misura che vanno oltre le aspettative dei clienti.
Una Visione Avanzata di Equità di Genere e Innovazione Tecnologica nel Cuore della Micronizzazione
Scopri come il Gruppo Munit ridefinisce il conditioning di prodotti micronizzati attraverso tecnologia avanzata e collaborazione.
Nel cuore di Fiorenzuola d’Arda, nel 1978, sorgeva un’azienda visionaria: Microchem. Fondata con una missione chiara – portare la micronizzazione dei principi attivi farmaceutici a nuove vette – Microchem ha iniziato il suo viaggio con
MUNIT, with its affiliates Jetpharma and Microchem, will be participating at the Formulation & Delivery UK .
The event will take place from 21 - 22 September 2021 in London (ILEC Conference Centre), UK + a digital day, 23 September (selected live broadcast
It is a story that started a long time ago, around the mid-sixties, when the micronization of active ingredients in pharmaceuticals was more or less unheard of.
In those days, fluid jet micronization was being used, in practice, more often in other sectors, for example in mineral, herbicide and antiparasitic products.
By following this principle and adapting it over the course of time to the increasing demands of the chemical and pharmaceutical industry, micronization started to take its first steps.
At first, the orders came from small and medium-sized
companies that found it difficult to set up departments soley for their micronization requirements, but then, in time, the first “blue chips” joined in.
With the consolidation of Microchem in 1978, and also at the Italian level, micronisation became fundamental. At that time Italy was the number one world market for the production of APIs and even today, it remains in third place, headed only by India and China.
Instilled in the company is the DNA of complementary skills held by its founder engineer, Alberto Martinoli: the art of designing and specifying micronization equipment and machinery and, on the other hand, the art of simply
raising service levels to an absolute maximum.
It is quality and service that are the focal points at Microchem: quality assurance, quality controls and careful laboratory analysis constitute the three pillars on which Microchem bases all of its micronization activities.
By service we mean the capacity to adapt the micronization specifications to the needs of the client, the constant presence of our staff and the flexible and dynamic approach that typifies the company culture at Microchem.
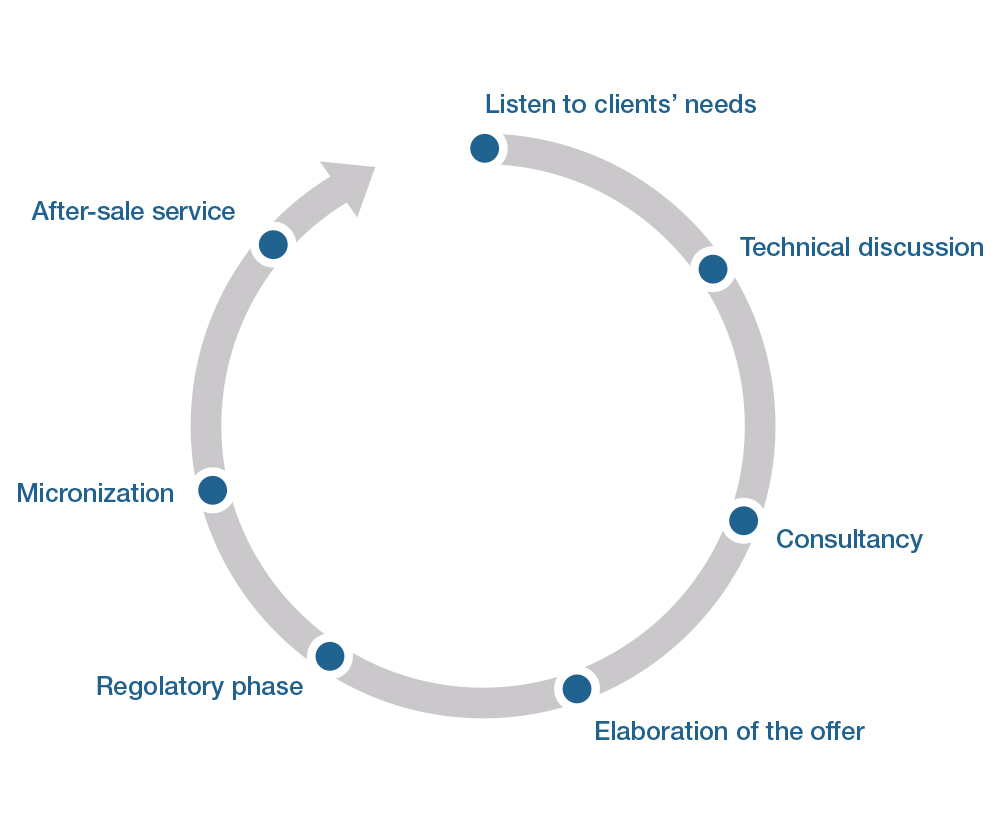
This scheme shows how Microchem works with its potential and existing clients. The relational aspect is really important for the company and all its employees. For us, quality means loyalty: it gives us enormous satisfaction to see how our clientele has remained loyal to us over the years, giving rise to long-lasting relationships and enabling us to create and build new profitability.

Scalability
Our spiral jet mills have been designed to guarantee precise scalability. This enables the active ingredient to be followed from its first R&D phases through to marketing; it allows us to operate with the same product from batches of 0.2 g up to several tonnes.
Extremely precise PSD
Thanks to our technology we are able to obtain very fine and precise particle size distribution with only a single micronization passage for nearly all pharmaceutical ingredients.
MICRONIZATION
Microchem provides contract micronization of active ingredients for the pharmaceutical industry. Micronization is undertaken using a range of fluid jet mills, conceived and designed by us.
Excellent Yield
We guarantee to reduce the product build-up and to achieve yields higher than 99% for the industrial process, starting from quantities of 1kg
No heat generation
Thanks to the isothermal feature of our jet mills, we can micronize any pharma ingredients, even those with a very low melting point (30-40°C).
DELUMPING
Spiral jet mills and a hammer mills can be used successfully not only to reduce the particle size, but also to remove lumps and agglomerates.
DISPENSING
Our clean rooms and isolators can be used to dispense and re-pack bulk API into small containers at any amount.



Microchem maintains and continuously seeks to improve its Integrated Quality, Safety and Environment Management System for the micronization services.
Microchem is in compliance with the current Italian regulations, which meet the requirements of good practice and quality controls recommended by ICH Q7, as well as the applicable laws on Health, Safety and Envionment.
Internal and external cGMP and ISO audits are constantly performed to ensure the continuous compliance to all applicable requirements.

ISO 8 (class 100.000) periodically qualified, designed to offer the best environment for the handling pharmaceutical products, avoiding environmental contamination and preventing product cross-contamination. Differential pressure cascade to obtain barrier effect and powder containment.
Microchem sustains an active management system in which it has integrated an occupational health and safety management, as to OHSAS 18001 standard.
Via Turati 2
I – 29017 Fiorenzuola d’Arda (PC)
GPS – 44.91855, 9.936372
P.Iva – CF – Reg. Imp 01026710333
R.E.A. PC 122206
Cap. Soc. € 93.600 i.v.
Subject to management and coordination
activities of AL.GA. Holding SA